how to name carbonyl groups Grupo carbonilo: nomenclatura y estructura, ejemplos, videos, preguntas
The carbonyl group is a functional group that consists of a carbon atom and an oxygen atom joined by a double bond. This group can be found in various compounds, including aldehydes, ketones, carboxylic acids, esters, and amides. Understanding the nomenclature and structure of carbonyl compounds is essential for professionals in fields such as chemistry, biochemistry, and pharmaceuticals.
Carbonyl Group Nomenclature and Structure
The nomenclature of carbonyl compounds follows a specific set of rules that dictate how these compounds are named. In general, aldehydes are named by replacing the final -e of the parent alkane with -al. For example, the aldehyde derived from ethane is called ethanal. Ketones, on the other hand, are named by replacing the -e of the parent alkane with -one. For instance, the ketone derived from propane is called propanone.
 The structure of the carbonyl group is composed of a carbon atom doubly bonded to an oxygen atom. This double bond gives the carbonyl group unique chemical properties, making it highly reactive and prone to participate in various types of chemical reactions.
The structure of the carbonyl group is composed of a carbon atom doubly bonded to an oxygen atom. This double bond gives the carbonyl group unique chemical properties, making it highly reactive and prone to participate in various types of chemical reactions.
Chemical Reactions of Carbonyl Compounds
Carbonyl compounds can undergo a wide range of chemical reactions due to the polar nature of the carbonyl double bond. Some important reactions include oxidation, reduction, nucleophilic addition, and condensation reactions.
Oxidation of primary alcohols results in the formation of aldehydes, which can be further oxidized to form carboxylic acids. Ketones, however, cannot be further oxidized due to the absence of an oxidizable hydrogen atom.
Reduction reactions, on the other hand, involve the conversion of carbonyl compounds into alcohols. This can be achieved using reducing agents such as sodium borohydride or lithium aluminum hydride.
Nucleophilic addition reactions are another important class of reactions involving carbonyl compounds. In these reactions, a nucleophile attacks the electrophilic carbon atom of the carbonyl group, resulting in the formation of a new bond. This is a common mechanism for the synthesis of organic compounds.
 Condensation reactions involving carbonyl compounds are often used in the synthesis of polymers, such as polyesters and polyamides. These reactions involve the formation of a new bond between the carbonyl group of one molecule and a nucleophile or electrophile of another molecule.
Condensation reactions involving carbonyl compounds are often used in the synthesis of polymers, such as polyesters and polyamides. These reactions involve the formation of a new bond between the carbonyl group of one molecule and a nucleophile or electrophile of another molecule.
Conclusion
Understanding the nomenclature and structure of carbonyl compounds is crucial for professionals working in chemistry-related fields. It allows them to accurately name and identify different types of carbonyl compounds, as well as predict their chemical behavior and reactions. With this knowledge, professionals can develop new compounds, design drugs, and contribute to advancements in various scientific and industrial applications.
If you are looking for Grupo carbonilo: nomenclatura y estructura, ejemplos, Videos, preguntas you’ve came to the right place. We have 5 Pics about Grupo carbonilo: nomenclatura y estructura, ejemplos, Videos, preguntas like Grupo carbonilo: nomenclatura y estructura, ejemplos, Videos, preguntas, 06.02 Nomenclature of Carbonyl Compounds - YouTube and also The Carbonyl Group - Chemistry LibreTexts. Here you go:
Grupo Carbonilo: Nomenclatura Y Estructura, Ejemplos, Videos, Preguntas
 belowzerofatfreezingclinic.comcarbonilo carbonyl nomenclatura miembros
belowzerofatfreezingclinic.comcarbonilo carbonyl nomenclatura miembros
9.1 Introduction & 9.2 Nomenclature Of Carbonyl Compounds - YouTube
 www.youtube.comcarbonyl nomenclature
www.youtube.comcarbonyl nomenclature
06.02 Nomenclature Of Carbonyl Compounds - YouTube
 www.youtube.comcarbonyl compounds nomenclature
www.youtube.comcarbonyl compounds nomenclature
Lecture For Lesson VI.1: Naming Carbonyl Compounds - YouTube
 www.youtube.comcarbonyl compounds naming
www.youtube.comcarbonyl compounds naming
The Carbonyl Group - Chemistry LibreTexts
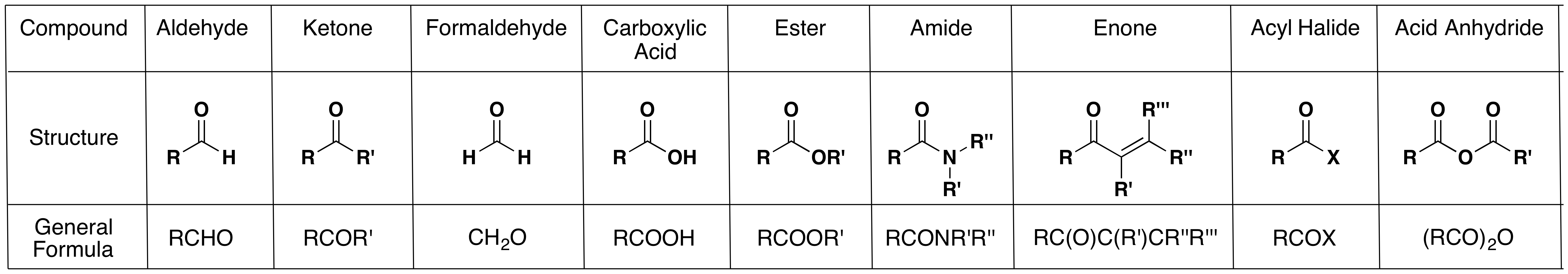 chem.libretexts.orgcarbonyl compounds group chemistry list organic properties chem ketones aldehydes unit some
chem.libretexts.orgcarbonyl compounds group chemistry list organic properties chem ketones aldehydes unit some
Carbonyl compounds nomenclature. Carbonilo carbonyl nomenclatura miembros. Carbonyl compounds group chemistry list organic properties chem ketones aldehydes unit some